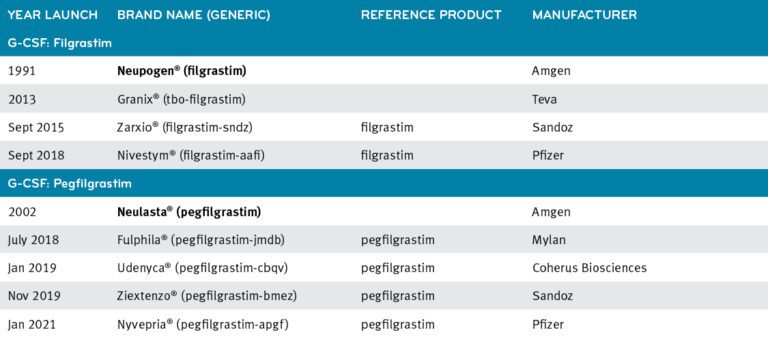
These highlights do not include all the information needed to use GRANIX safely and effectively. See full prescribing information for GRANIX.GRANIX® (tbo-filgrastim) injection, for subcutaneous use Initial U.S. Approval: 2012

Filgrastim-Teva® seringues prêtes à l'emploi 30 MIU/0,5 ml et 48 MIU/0,8 ml sont remplacées à la PHEL par Zarzio® seringues